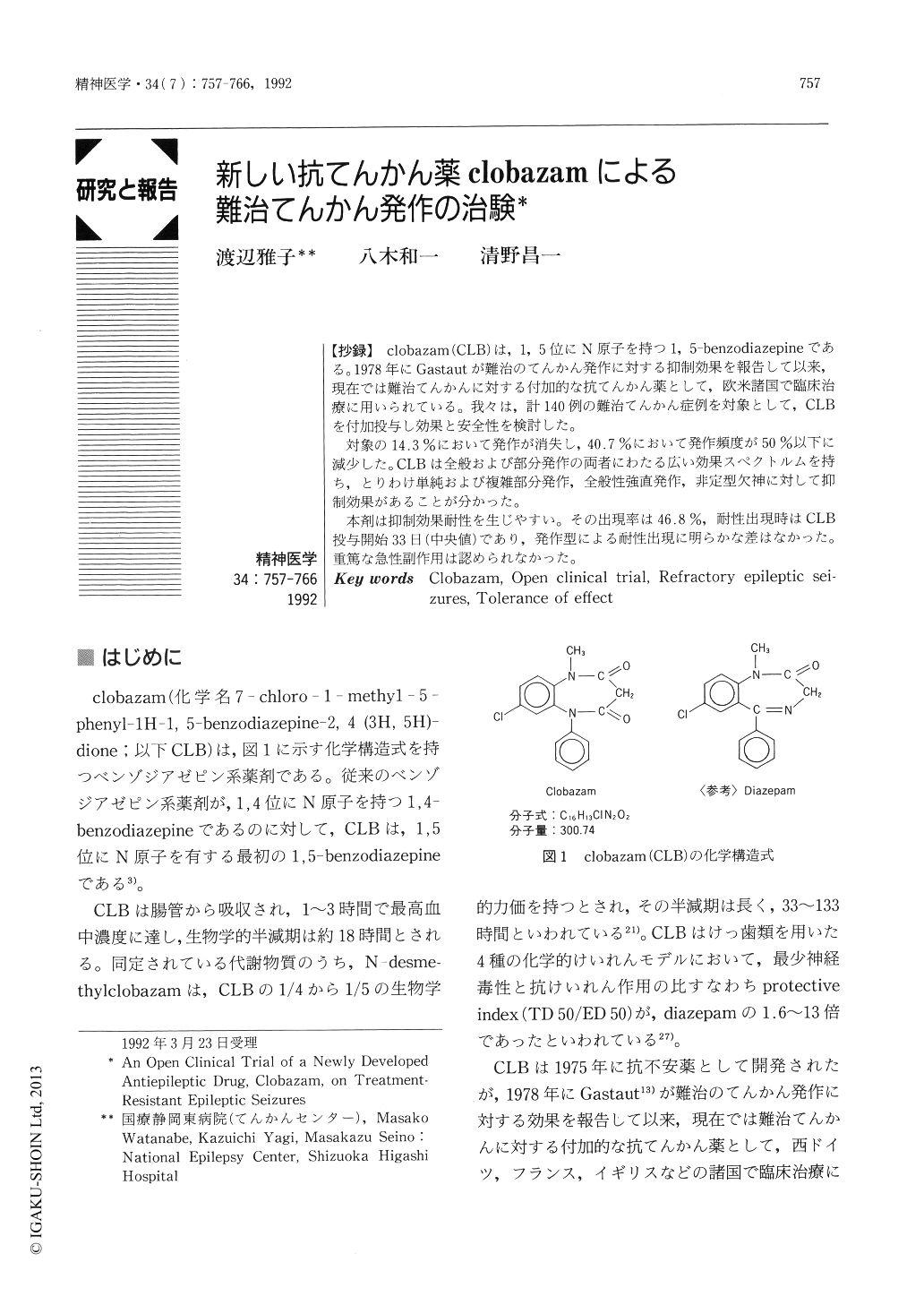
Japanese
English
- 有料閲覧
- Abstract 文献概要
- 1ページ目 Look Inside
【抄録】 clobazam(CLB)は,1,5位にN原子を持つ1,5-benzodiazepineである。1978年にGastautが難治のてんかん発作に対する抑制効果を報告して以来,現在では難治てんかんに対する付加的な抗てんかん薬として,欧米諸国で臨床治療に用いられている。我々は,計140例の難治てんかん症例を対象として,CLBを付加投与し効果と安全性を検討した。
対象の14.3%において発作が消失し,40.7%において発作頻度が50%以下に減少した。CLBは全般および部分発作の両者にわたる広い効果スペクトルムを持ち,とりわけ単純および複雑部分発作,全般性強直発作,非定型欠神に対して抑制効果があることが分かった。
本剤は抑制効果耐性を生じやすい。その出現率は46.8%,耐性出現時はCLB投与開始33日(中央値)であり,発作型による耐性出現に明らかな差はなかった。重篤な急性副作用は認められなかった。
Clobazam is a 1,5-benzodiazepine having nitrogen in the 5-position of the heterocyclic ring and, since Gastaut's report on the therapeutic effect of this compound on refractory epileptic seizures (1978), has been used in Europe as an antiepileptic agent for treatment-resistant epileptic patients. We investigated the effect as well as the safety of clobazam as an adjunctive drug on a total of 140 patients with refractory seizures insufficiently controlled by preexisting antiepileptic drugs.
In 14.3 % of the patients, their seizures were completely controlled, and a greater than 50 %decrease in seizure frequency was achieved in 40.7 % of these patients. Clobazam was found to have a broad antiepileptic spectrum for both partial and generalized seizures, in particular, for simple and complex partial seizures, generalized tonic seizures, and atypical absence seizures.
The development of tolerance to Clobazam was found in 46.8 % of the patients. The relapse of hitherto controlled seizures took place on anaverage of 33 days after the initiation of treatment using clobazam (median). There was no definitive difference in the duration of the clobazam effect due to seizure types. Clobazam was revealed to produce no severe adverse effects, and was found safe for clinical use.
Copyright © 1992, Igaku-Shoin Ltd. All rights reserved.


